First-in-class drug candidates able
to restore insulin sensitivity
with anti-inflammatory and
anti-adiposity actions.
Unique and innovative mechanism of action

THAC, The Healthy Aging Company, is a US-based privately-owned biopharmaceutical company which has the ambition to develop a range of
first-in-class drug candidates targeting T2DM and its complications.
Its pipeline could lead to potential real game changers for patient care and disease management. THAC is primarily focused on Type 2 Diabetes Mellitus, a condition with still unmet medical needs.
THAC drug candidates have a unique and innovative mechanism of action that targets oxidation and inflammation, which restores the composition of microbiota, and the insulin sensitivity.
THAC envisions that by fighting insulin resistance, the root cause of T2DM, major breakthrough could be achieved for the treatment of T2DM and the prevention of severe related complications such as peripheral neuropathy (e.g. diabetic foot wounds, amputations).
THAC drug candidates are intended to delay disease progression, improve patient’s quality of life, delay premature death and prevent T2DM complications; peripheral neuropathy is a severe complication for which there is no treatment available, e.g., diabetic foot wounds and amputations.
THAC builds a pipeline of game changer therapies and develops a range of first-in-class drugs for T2DM patient’s care and disease management
THAC is a clinical stage company focused on research, development and commercialization of game changer therapies for T2DM and speed up its preclinical and clinical study programs by strongly invest.
The unique and innovative mechanism of action of ALF-5755 allows to restore insulin resistance, the root cause of T2DM disease, as well as the microbiota composition. This mechanism of action and all its scientific and preclinical results already observed are the object of publications in high level scientific journals.
Regulatory preclinical studies were performed and the safety in humans was confirmed in a successful first-in-man phase I clinical study.
THAC is now in the process of launching a phase II clinical study. In parallel, we anticipate phase I bridge studies with different formulations, specifically designed to restore gut microbiota composition and to further improve T2DM patient management.
THAC concluded an exclusive global licensing agreement with a partner for the commercialization and development rights of the molecule in this indication. THAC will conclude new partnerships with biopharmaceutical industry leaders at the end of phase II studies.
ALF-5755, a First-in-Class drug candidate for the treatment of T2DM and its complications
Derived from a human endogenous protein
Which is secreted under inflammatory conditions, mainly by the Paneth cells (intestine)
And which belongs to the archaic innate immune system in order to defend the body against aggression and to promotes the glucidic and lipidic homeostasis.
ALF-5755 has a unique and innovative mechanism of action
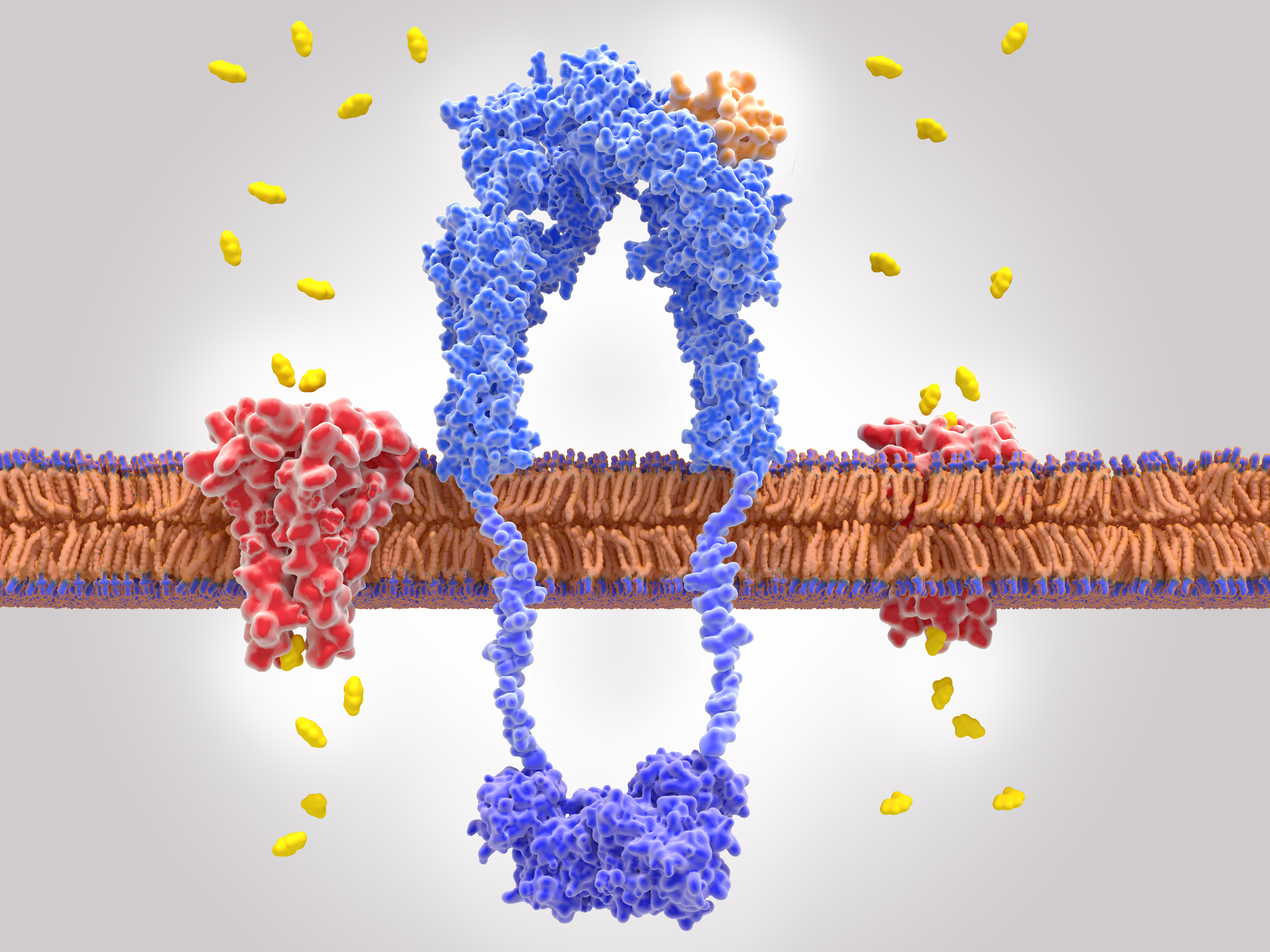
The active ingredient of THAC drug candidates has intrinsic ROS scavenger and anti-inflammatory properties when injected in the body and after binding to the fibrin network of the extracellular matrix of the inflamed tissue.
ALF5755 binds the fibrin molecule in the extracellular matrix in an environment of inflammation (Moniaux ., Bréchot C., PLoS 2016).
This binding leads to a local anti-inflammation and ROS-scavenger effects which induce intracellular signaling pathway, the activation of the receptor of insulin and the receptor of glucose.
Its properties induce a combination of biological actions, including restoration of the insulin sensitivity, anti-inflammatory and anti-adiposity effects.
The unique and innovative mechanism of action of the active substance has been published by the team in high level scientific journals.
An active substance which acts on the peripheral neuropathy, a severe T2DM complication with no treatment
A unique active substance which leads to restore the insulin sensitivity, the root cause of T2DM, promoting glucose uptake in skeletal muscles.

♦ Peripheral neuropathy is one of the most common severe complications of T2DM. It leads to foot wounds and ulcers which progresses to amputations.
♦ Up to 30% of T2DM patients develop this severe complication.
♦ Do you know that T2DM is the first cause of amputation in the world?
♦ Despite the severity of the complication, there is no treatment.
A strong patents portfolio and know-how for a robust protection
♦ Several family patents have already been granted to protect THAC drug candidates in different diseases and territories, focused on insulin resistance and microbiota composition.
♦ Other patents are pending.
♦ A solid know-how has been acquired for ALF-5755 production.